VCU Health leads clinical trial of advanced artificial heart
VCU Medical Center is one of seven U.S. clinical trial sites for CARMAT — the world's most advanced artificial heart.
November 25, 2020
 Most patients with advanced heart failure can be managed by a left ventricular assist device (LVAD). But some have no option but a heart transplant or the current version of a total artificial heart.
Most patients with advanced heart failure can be managed by a left ventricular assist device (LVAD). But some have no option but a heart transplant or the current version of a total artificial heart.
Heart surgeon Dr. Vigneshwar Kasirajan, chair of the VCU Health Department of Surgery, is principal investigator of a clinical trial to determine the safety and efficacy of a new, improved version of the total artificial heart. The trial will begin once FDA approval is secured — perhaps this winter. Here, Dr. Kasirajan provides the details.
What is CARMAT?
CARMAT is the name of the new artificial heart. It is named after the company and the two individuals who developed it. The CARMAT device replaces both of the lower pumping chambers of the heart. It's very similar to the artificial heart we have right now, made by SynCardia, but there are key differences.
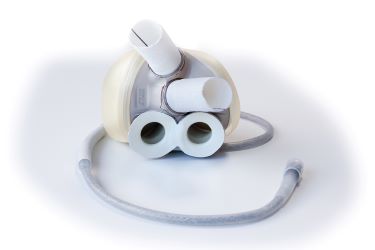
The CARMAT total artificial heart.
What are the differences?
Our current artificial heart is pneumatically (air) powered through a console the patient wears in a backpack. The new heart features two small motors tucked inside the device. It's powered by external, wearable batteries that can be hidden underneath a jacket. It's totally implantable, with only a small driveline. It's also quite a bit quieter — nearly silent.
The current artificial heart also uses mechanical valves and requires the patient to take blood thinners — which increase the risk of bleeding. The new heart is made of biological materials, which we believe will reduce the need for blood thinners. That's one of the things we'll be studying in the trial.
Overall the design is a big step forward.
What's the goal of the trial?
This is a trial to prove that the CARMAT total artificial heart can meet FDA standards for performance and safety.
Why is this trial significant?
In the U.S., the total number of heart transplants done per year is approximately 3,000. The total number of patients per year who would benefit from heart replacement is probably 50,00-60,000.
No matter how hard we work to increase the number of donors available for heart transplantation, that gap is too broad. We need an off-the-shelf device that could be used for the variety of patients, such as different sizes, different ages, and would produce good outcomes, similar to a heart transplantation.
I think in another five to 10 years, we will make multiple modifications to the CARMAT device to benefit a number of patients with advanced heart disease who could have a pump put in and maintain a good quality of life. That's what we want to get to.
How did VCU Health become a test site?
VCU Health has a lot of experience with artificial hearts, and we have had some of the best outcomes in the world. CARMAT came to us because of our experience with artificial heart technology, patient management, patient selection and good outcomes.
Who could benefit from the CARMAT total artificial heart?
The CARMAT heart is meant for patients with advanced heart failure who require a heart transplant. So while they're waiting, if they get very sick, they could potentially go to the CARMAT artificial heart and then go towards the transplant. Once the pump is in, it will stabilize them. A drawback of the device is that it's fairly big. It doesn't fit everybody. So, the device would not fit a smaller woman.
Who can participate in the trial?
We plan to enroll 10 patients at seven different heart centers across the country.
There are a lot of patients who could benefit from the total artificial heart, but because this is new technology, we are restricting the trial to a small group to allow us to study their outcomes carefully before we expand its use.
Participants will be patients who we evaluate for heart transplantation, and they may have severe biventricular failure. They are not going to be able to wait for a heart for many months. They might need an LVAD (left ventricular assist device) to take them to a heart transplant safely.
We screen patients for the trial every day. We're the largest heart transplant program in Virginia, and I think we'll see a lot of patients being referred to us.
Describe the timeline.
It'll take probably two to three years to enroll enough patients, as our enrollment criteria are quite strict. For instance, we are looking for patients who are ill, but not so ill that they might die on the device. We are also looking for people who are the right size, so the device will fit well inside them. Patients also need financial and social support, among other criteria.
Our goal for the trial is to get the patients we implant with the device on to transplantation or to survival up to six months.
What does hosting this trial mean to VCU Health?
VCU Health is focused on innovation to benefit patients. We're constantly looking at new ways to make life better for them. That can be done only by working together in large teams, in a complex system where we can partner with others to create new knowledge and new technology. There are lots of things we need to learn as we go forward, but as an institution and as a heart center, to be able to be a part of a large trial like this, to help patients in the future — this trial is a very exciting step for us.
If someone thinks they may be a good candidate, how do they enroll?
If you think you may be a good candidate, speak with your cardiologist. We anticipate receiving referrals to our heart transplant and heart failure team from colleagues around the country.
For more information
To learn more about the CARMAT total artificial heart, visit the CARMAT website. To learn more about the VCU Pauley heart failure program, please visit the Pauley Heart Center.