VCU-created Nerve Tape device gets FDA 510(k) Clearance
The suture-less solution for surgical repair is anticipated for patient use in 2023
August 22, 2022
By Jeff Kelley for VCU Innovation Gateway
Nerve Tape, a suture-less solution for surgical repair of transected nerves created by a Virginia Commonwealth University orthopaedic surgeon, has received clearance from the U.S. Food and Drug Administration under its 510(k) Clearance process.
The step means that Nerve Tape's development partner, Atlanta-based BioCircuit Technologies, can market the product in anticipation of the first human use in 2023.
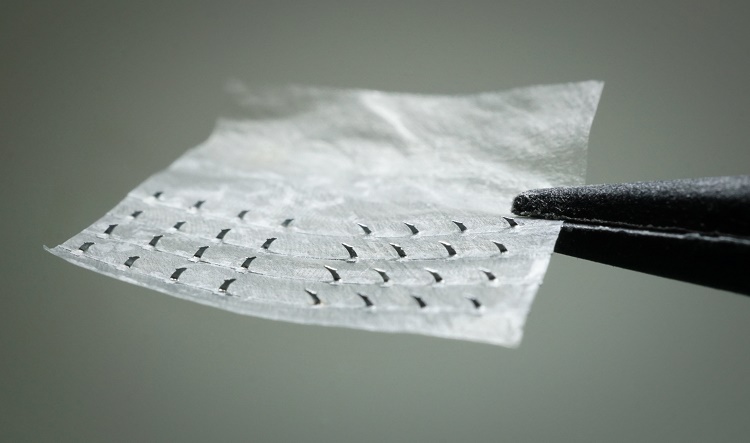 Nerve Tape is a device consisting of microhooks embedded in a biologic wrap. The tiny hooks bind firmly safely, and reversibly to the nerve's outer connective tissues to align peripheral nerves following traumatic injuries. For patients with nerve injuries, the tape can ultimately lead to better nerve regeneration and clinical recovery.
Nerve Tape is a device consisting of microhooks embedded in a biologic wrap. The tiny hooks bind firmly safely, and reversibly to the nerve's outer connective tissues to align peripheral nerves following traumatic injuries. For patients with nerve injuries, the tape can ultimately lead to better nerve regeneration and clinical recovery.
"The development and clearance of Nerve Tape represents a significant advancement in the treatment of nerve injuries," said Nerve Tape inventor Jonathan Isaacs, M.D., professor and chair in the Division of Hand Surgery at VCU Medical Center. "This product has the potential to offer surgeons a faster, simpler method for achieving a precise, reliable repair of injured nerves. As a co-inventor with several years of experience using the device in animal models, I look forward to having Nerve Tape available for clinical use."
As VCU's technology transfer office, "we are thrilled by the FDA clearance of Nerve Tape and hope it could get to market and help patients as quickly as possible," said Ivelina Metcheva, Ph.D., assistant vice president for innovation at VCU Innovation Gateway. "As a practicing surgeon, Dr. Isaacs is a great role model for other physicians as proof that inventions are not just created in laboratories but could be made in a real-world health care setting."
BioCircuit, which licensed Nerve Tape in 2016, is working with supply partners in preparation for the U.S. launch. Michelle Jarrard, BioCircuit's CEO, called the clearance a "critical milestone" toward providing a new treatment for peripheral nerve injuries.
Nerve Tape is the company's first FDA-cleared medical device and "exemplifies our commitment to equipping surgeons with powerful, practical tools to improve the treatment of injuries, and we are excited to be entering the commercial phase of development as we prepare to bring this solution to market," she said.
BioCircuit has received generous funding from numerous NIH grants. In addition to ongoing grant support, BioCircuit has attracted private financing, including investment from the GRA Venture Fund, Masters Capital and Alsora Capital.
Read more on Nerve Tape in Innovation Gateway's 2021 Annual Report.




