Deep Brain Stimulation
The deep brain stimulation procedure generates electrical impulses that disrupt abnormal brain signals in patients with movement disorders, such as Parkinson’s disease.
Deep brain stimulation is a surgical intervention used to treat movement disorders such as dystonia, essential tremor and Parkinson’s disease when the regimen of existing medications and the various rehabilitation strategies become less effective in managing symptoms. This is a relatively new surgical procedure that received approval from the Food and Drug Administration to treat essential tremor and tremor in Parkinson’s disease in July 1997 and for advanced motor symptoms of Parkinson’s disease in January 2002. It is currently approved for the treatment of dystonia through a “humanitarian device exemption.”
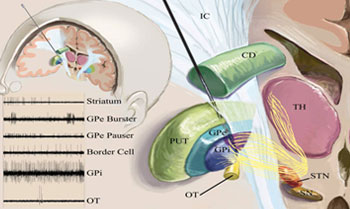
The subthalamic nucleus (STN), the globus pallidus (GPi) and the thalamus are three locations in the brain that are targeted in the DBS procedure for the treatment of movement disorders. DBS administers a well-controlled electrical current into the target area(s). This electrical current functions as an “off switch” by disrupting abnormal brain signals responsible for the abnormal physical movement. This disruption helps restore normal activity in the brain thus enabling more controlled movement. DBS does not involve destruction of brain tissue and its effects are reversible and adjustable. It is now preferred over the thalamotomy or pallidotomy, two surgical techniques that involve the actual destruction of the brain cells that are “misfiring.” Electrical impulses are generated from an implanted battery and then pass through the lead and into the target area. This entire system is implanted under the skin.
Accurate placement of brain leads or wires is necessary for effective treatment through DBS.
The effectiveness of the DBS procedure depends on accurate placement of the brain lead(s) or wire(s) and therefore requires special expertise. The evaluation and surgical procedure are conducted at VCU Medical Center using a team approach that involves neurosurgery, neurology, neuropsychology and nursing staff. The target areas or nuclei are quite small and special imaging techniques and guidance devices are used to help position the leads. In addition, certain areas of the brain have a characteristic sound. Microelectrode recording (MER) is conducted during DBS surgeries of the STN and GPi and involves “listening” to the brain cells to help identify these specific areas. The patient is awake during this and other portions of the surgery and becomes an important member of the team. The patient also helps determine whether beneficial effects occur when the stimulation is applied during surgery.
DBS can be quite an effective treatment in hard-to-treat patients who meet specific criteria. However, there is a one to three percent risk that the brain will be injured during the DBS procedure. As a result of this damage, patients may experience loss of speech, paralysis, coma or even death – usually caused by bleeding in the brain. There is an additional five percent risk of infection that usually requires the removal of the device. DBS surgery can also be time consuming, usually lasting three to four hours per side of the brain being operated on. The most common complaints from patients include back and neck pain and fatigue. These complaints occasionally can become so disabling that the patient has a difficult time participating in the surgery and/or requests to have the surgery prematurely stopped.
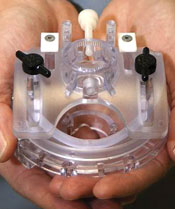
New frameless technology allows DBS patients to receive treatment without the mobility impairments associated with the traditional frame approach.
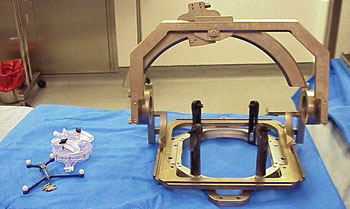
The traditional approach to DBS surgery involves attaching a large, metal halo device to the patient’s skull and securing it to the surgical table. The frame’s effectiveness has been proved during several decades of use. However, the negative aspects of the frame are that it creates complete immobilization of the patient’s head and neck and it obscures the patient’s line of vision.
The NexFrame System offers advanced treatment for movement disorders, as well as epilepsy and depression.
In an effort to simplify the DBS procedure and enable greater patient comfort and participation during surgery, neurosurgeons at VCU, Cleveland Clinic and Tulsa, Okla., worked with Image Guided Neurologics Inc. to develop a frameless stereotaxy technique. In this technique, the heavy frame has been replaced with five small bone screws and the NexFrame, a disposable guidance device. This new device does not require that the head and neck be kept in a fixed position and the patient can move or adjust his or her position if needed. Importantly, the accuracy of the frameless and framed techniques has been found to be equal. Dr. Kathryn Holloway performs frameless DBS at VCU Medical Center and at McGuire VA Medical Center in Richmond, Va.
In August 2005, Medtronic Neurological, the world-wide leader in DBS therapy, acquired IGN and the NexFrame technology. The implications of DBS therapy are far-reaching not only in the treatment of movement disorders but for disorders such as epilepsy and depression. DBS and this new frameless option offer much promise for those suffering from various movement disorders that are refractory to medical therapies.