Recruiting is underway for ADVENT clinical trial
VCU Medical Center is one of 37 participating locations testing Farapulse Inc.'s new pulse-field ablation device
More than a year ago, Kenneth Ellenbogen, M.D., tested Farapulse Inc.’s new pulse-field ablation (PFA) device in its pre-clinical trial phase. Impressed by its safety and effectiveness, Ellenbogen, director of clinical cardiac electrophysiology and pacing at VCU Medical Center, knew the device would forever change the practice of ablation.
Today, Ellenbogen is a principal investigator for the ADVENT clinical trial to test the PFA against standard ablation methods. VCU Medical Center is one of 37 participating locations for the trial, which started this spring and will include about 350 patients.
Atrial fibrillation is a heart rhythm disorder that affects nearly 6 million Americans and makes them five times more likely to have a stroke than individuals with a regular heartbeat. When an abnormal heartbeat is not responsive to medication, doctors often recommend ablation to restore the heart to its normal rhythm. To stop the transmission of erratic electrical impulses, doctors apply extreme cold or heat to the spasming heart tissue using a catheter guided into the heart through various blood vessels. This scars the tissue and prevents it from transmitting electrical impulses to the rest of the heart, returning the heart to its normal rhythm and function.
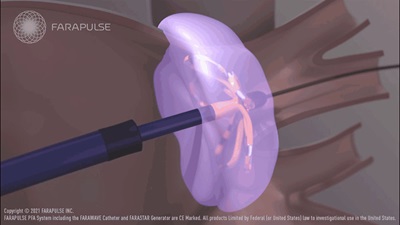
But applying extreme cold or heat to tissue inside the heart can sometimes damage healthy tissue in surrounding areas, such as the esophagus or major nerves. To address this need for a more accurate ablation technique, Farapulse created a new pulse-field ablation device that uses a localized electrical field to scar the spasming heart tissue. Based on consistent early data, PFA does not cause collateral damage to the surrounding tissue.
While traditional ablation methods including radiofrequency or cryoballoon cannot be focused enough to eliminate risk to normally functioning tissue, Farapulse’s PFA reduces the treatment area in such a way that providers can treat very specific areas of cardiac tissue, leaving surrounding tissue unchanged. This is accomplished by conducting electricity through a specialized attachment at the end of a catheter. After the catheter is inserted into the heart, the strands of the attached conductor can be pushed out to form a bulb, and then flattened in the shape of a flower to better contact the tissue being treated.
With traditional methods of ablation, treatment of the affected tissue can take up to 30 minutes. But with Farapulse’s PFA, tissue can be treated in a matter of seconds. A provider can ablate the left atrium and pulmonary veins in 15 minutes, and recovery times remain unchanged.
Learn more about the FARAPULSE ADVENT clinical trial.
Back to Autumn-2021

Join our Pauley Consortium composed of patients, friends and advocates.

