History of severe allergic reactions? This COVID-19 vaccination study may be for you
Unvaccinated people, including those with a history of severe allergic reactions, can join a clinical trial of COVID-19 vaccination at VCU Health
July 08, 2021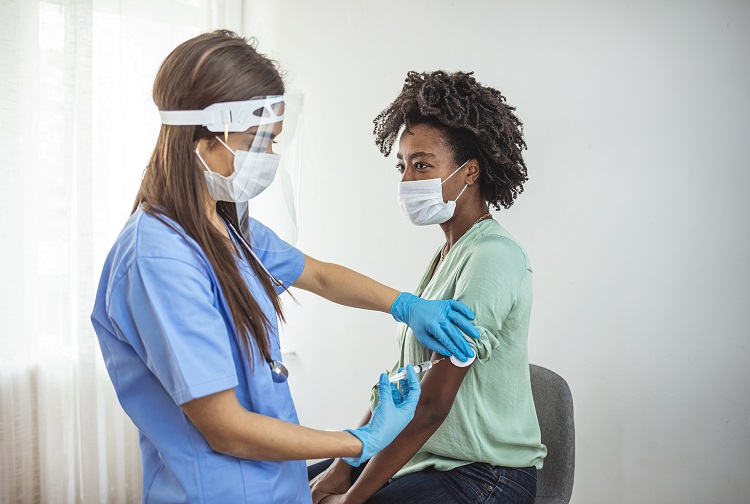 Photo: Getty Images
Photo: Getty Images
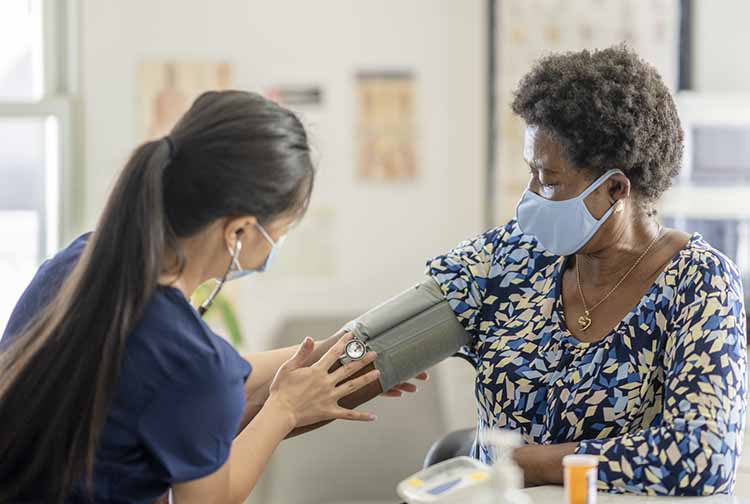
Individuals with a reported history of severe allergic reactions to foods, medications, radiocontrast dyes, insect stings or vaccines may be eligible for a national study of COVID-19 vaccinations at VCU Health.
Through this nationwide clinical trial, sponsored and funded by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health, VCU Health will offer people with a history of severe allergic reactions or a mast-cell disorder an opportunity to receive a COVID-19 vaccine, along with a monitoring period overseen by an allergy team and additional contact with the study’s team after receiving the vaccine.
Lawrence Schwartz, M.D., Ph.D., chief of the Division of Rheumatology, Allergy and Immunology in the Department of Internal Medicine at VCU Health, is VCU Health’s site leader of the “Systemic Allergic Reactions to SARS-CoV-2 Vaccination” clinical trial.
The study will see if people who are highly allergic or have a mast cell disorder are at increased risk for systemic allergic reaction to the Moderna or Pfizer-BioNTech COVID-19 vaccines. The study will also investigate whether certain factors increase risk of an allergic reaction to these vaccines and attempt to identify the cause of such reactions to better enable their treatment or prevention.
The study is open to unvaccinated people ages 12-69 years with a history of severe allergic reactions or a diagnosis of a mast cell disorder. People with no history of allergic reactions and no history of a mast cell disorder can join the study’s control group.
On the first visit, participants may receive either a dose of placebo or a vaccine. Study participants will be monitored for a period of 90 minutes after each injection, longer than the standard 15-minute observation. By the end of the study, all participants, including those who originally received a placebo, will receive two doses of either the Moderna or the Pfizer COVID-19 vaccine.
Offering people with a history of severe allergic reactions or a mast-cell disorder a chance to receive a COVID-19 vaccine in the study’s controlled environment in the presence of physicians, nurses, and clinical research coordinators aligns with VCU Health’s goal to provide the highest quality experience and safest healthcare to all.
VCU Health is one of two sites in Virginia and one of 29 sites across the country taking part in this study.
If you are interested in learning more about this study, contact study coordinator Esperanza Gilbert at esperanza.gilbert@vcuhealth.org or leave a message with contact information at (804) 573-9161.