Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I'm Dr F. Perry Wilson.
When it comes to sepsis and septic shock, docs are used to disappointment. When I was training, the new hotness was stress-dose steroids and dual coverage for gram-negatives, then Rivers' early goal-directed therapy, then activated protein C. Time and again, early dramatic successes gave way to less impressive or negative studies, and the hope that there was a silver bullet for sepsis diminished.
But one therapy has proven tantalizingly resilient, not only for reports of dramatic successes but also because of its low cost and virtual absence of side effects. That therapy? Vitamin C.
In June 2017, Dr Paul Marik published an observational before/after study in Chest that many in the community called simply too good to be true.
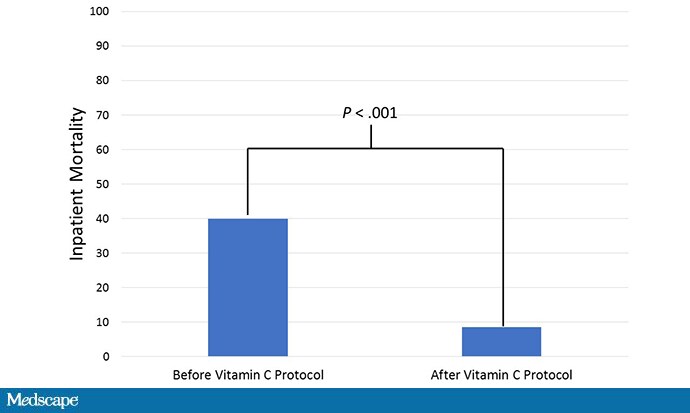
Forty-seven patients with sepsis before institution of a vitamin C–based protocol: mortality rate, 40%.
Forty-seven patients after institution of a vitamin C protocol: mortality rate, 8.5%. Not a randomized trial, but crazy good results.
I spoke with Dr Marik, asking how things have gone since he published the Chest paper.

But the lack of a randomized trial was a real blemish in this area of research. Skeptics noted that before/after studies are uniquely susceptible to selection bias, even cherry-picking. Show us the evidence.
Well, the evidence may be here, though it's not all we would have hoped for.
The CITRIS-ALI trial[1] appeared in JAMA this week. It enrolled 167 patients with sepsis and acute respiratory distress syndrome, and randomized them to high-dose IV vitamin C 50 mg/kg every 6 hours for 96 hours versus placebo in a double-blind fashion. The primary outcome was not mortality but rather changes in inflammatory biomarkers and SOFA (Sequential Organ Failure Assessment) score. I asked lead author Dr Berry Fowler: Why the surrogate outcomes?

In other words, this was going to be a small study. You'd therefore need a big difference in a hard outcome like mortality to achieve statistical significance. They played it safe and looked at changes in continuous metrics like SOFA score for their primary outcome.
The team may be regretting that decision. At 96 hours, there was no difference in SOFA score, C-reactive protein, or thrombomodulin levels in the two groups.

Primary outcome: negative, no effect of vitamin C.
Except that, oh yeah—there were fewer deaths in the vitamin C arm: 30% mortality versus 46% in the placebo arm.

To quote Dawn from the hit Broadway musical Waitress, "What do I do with that?"
I asked Dr Fowler. Did he consider this study a success?

Why can't we just say, "Look, significantly lower mortality in the vitamin C arm! Who cares about the semantics of primary and secondary outcomes?"
The reason why this is an issue becomes clear if you imagine that the opposite outcome occurred: no difference in mortality between vitamin C and placebo. Because mortality was a secondary outcome, the authors could legitimately say, "We didn't power the study for mortality. This was a small study looking at intermediate outcomes." And we'd all be happy with that. In other words, if we embrace the mortality outcome here, we're basically saying there's no way to lose with this study design. Significant difference in mortality? Sing it from the rooftops. No difference in mortality? Oh well, it was a secondary outcome anyway.
So, back to my question: What do we do?
The pat answer is call for more research. If nothing else, the vitamin C researchers have earned themselves the right to (and hopefully the funding for) a truly large-scale study.
But what do we do in the meantime? If this were a drug with big risks, or if it were particularly expensive, I'd do the slow medicine thing—wait for more info.
But it's not expensive. And it's not that risky. The nephrologist in me was worried about oxalate nephropathy, but apparently that hasn't emerged. Dr Fowler pointed out to me that IV vitamin C causes the point-of-care glucose checks to read too high—helpful FYI there.
But basically, this is a pretty low-risk intervention. It's easy for me to sit here and say, "Unless the patient has scurvy, wait for more data." But if a loved one was in the ICU with sepsis, would you ask for vitamin C? After reviewing the literature to prepare for this commentary, I'd be hard-pressed to dismiss it out of hand.
Follow Medscape on Facebook, Twitter, Instagram, and YouTube
Medscape © 2019 WebMD, LLC
Any views expressed above are the author's own and do not necessarily reflect the views of WebMD or Medscape.
Cite this: Does Vitamin C in Sepsis Live Up to the Hype? - Medscape - Oct 02, 2019.












Comments